Working with TCGA data: clinical, expression, mutation and methylation
Introduction
TCGA, The Cancer Genome Atlas, assembles multi-omic data on many tumor samples.
We will discuss how to work with data acquired using the RTCGAToolbox package. A substantial part of the effort of illustrating this system resides in the downloading of large resources from the archive. The vignette for the toolbox package describes high-level utilities for analysis; here we’ll focus on data harmonization and manual analysis.
Here’s an illustration of the download effort for three data types in rectal adenoma:
library(ph525x)
firehose()

The integrative object
We used the commands
library(RTCGAToolbox)
readData = getFirehoseData (dataset="READ", runDate="20150402",forceDownload = TRUE,
Clinic=TRUE, Mutation=TRUE, Methylation=TRUE, RNASeq2GeneNorm=TRUE)
which takes about 10 minutes to acquire and save on a good wireless connection. The show method for the object prints
readData
## READ FirehoseData objectStandard run date: 20150402
## Analysis running date: 20160128
## Available data types:
## clinical: A data frame of phenotype data, dim: 171 x 22
## RNASeq2GeneNorm: A matrix of count or normalized data, dim: 20501 x 105
## Methylation: A list of FirehoseMethylationArray object(s), length: 2
## GISTIC: A FirehoseGISTIC for copy number data
## Mutation: A data.frame, dim: 22075 x 39
## To export data, use the 'getData' function.
and hides the dimensionalities of the two methylation assays. These can be found via
> lapply(readData@Methylation, function(x) dim(x@DataMatrix))
[[1]]
[1] 27578 76
[[2]]
[1] 485577 109
A view of the clinical data
A complete understanding of the dataset would require attention to the method by which the “cohort” of tumors was assembled, including establishment of a common time origin for disease progression or death event times, along with details on tumor sampling and assay procedures. For the purposes of this course, we’ll assume that we can meaningfully combine all the data that we’ve retrieved.
Selecting a severity measure
clin = getData(readData, "clinical")
names(clin)
## [1] "Composite Element REF"
## [2] "years_to_birth"
## [3] "vital_status"
## [4] "days_to_death"
## [5] "days_to_last_followup"
## [6] "primary_site_of_disease"
## [7] "neoplasm_diseasestage"
## [8] "pathology_T_stage"
## [9] "pathology_N_stage"
## [10] "pathology_M_stage"
## [11] "dcc_upload_date"
## [12] "gender"
## [13] "date_of_initial_pathologic_diagnosis"
## [14] "days_to_last_known_alive"
## [15] "radiation_therapy"
## [16] "histological_type"
## [17] "radiations_radiation_regimenindication"
## [18] "completeness_of_resection"
## [19] "number_of_lymph_nodes"
## [20] "race"
## [21] "ethnicity"
## [22] "batch_number"
The severity of the disease will be indicated in pathology variables. T staging refers to size and invasiveness of tumor, N staging refers to presence of cancer cells in various lymph nodes.
with(clin, table(pathology_T_stage, pathology_N_stage))
## pathology_N_stage
## pathology_T_stage n0 n1 n1a n1b n1c n2 n2a n2b nx
## t1 8 1 0 0 0 0 0 0 0
## t2 28 3 0 1 0 0 0 0 0
## t3 49 30 3 4 1 22 2 2 2
## t4 2 1 0 0 0 2 0 0 0
## t4a 1 1 0 0 0 1 0 5 0
## t4b 0 0 0 0 0 0 1 0 0
We see that there is variability in both staging measures. We’ll reduce the T staging to avoid small class sizes.
clin$t_stage = factor(substr(clin$pathology_T_stage,1,2))
table(clin$t_stage)
##
## t1 t2 t3 t4
## 9 32 115 14
Defining survival times
We’ll guess that the vital status variable corresponds to status at last followup and that the days to last followup are recorded from a common origin in the diagnostic process. Patient presents, tumor is graded and staged, and the followup calendar begins.
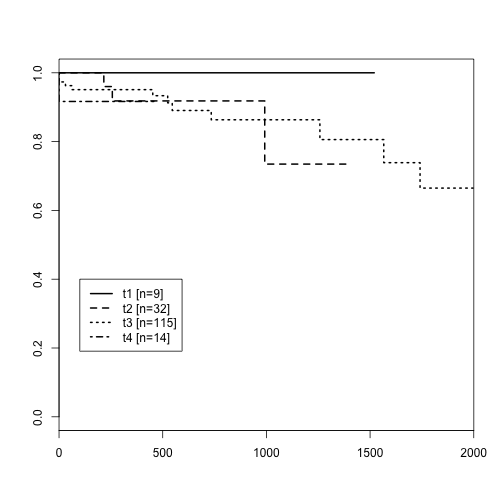
The following Kaplan-Meier display is a crude sanity check, showing that tumor stage 1 has no observed events, stages 2 and 3 are ordered as we would expect for the first 1000 days or so, and then the curves cross; the data are sparse.
library(survival)
ev = 1*(clin$vital == 1)
fut = as.numeric(clin$days_to_last_followup)
su = Surv(fut, ev)
plot(survfit(su~t_stage, data=clin), lwd=2, lty=1:4, xlim=c(0,2000))
ntab = table(clin$t_stage)
ns = paste("[n=", ntab, "]", sep="")
legend(100, .4, lty=1:4, lwd=2, legend=paste(levels(clin$t_stage), ns))
Introducing mutation data
mut = getData(readData, "Mutation")
dim(mut)
## [1] 22075 39
table(mut$Variant_Classification)
##
## 3'UTR 5'Flank 5'UTR
## 11 3 14
## De_novo_Start_InFrame De_novo_Start_OutOfFrame Frame_Shift_Del
## 4 31 176
## Frame_Shift_Ins IGR In_Frame_Del
## 161 24 30
## In_Frame_Ins Intron Missense_Mutation
## 8 132 14767
## Nonsense_Mutation Nonstop_Mutation Read-through
## 1964 6 10
## RNA Silent Splice_Site
## 16 4675 43
Let’s order genes by the number of missense or nonsense mutations recorded.
gt = table(mut$Hugo, mut$Variant_Classification)
mn = apply(gt[,12:13], 1, sum)
omn = order(mn, decreasing=TRUE)
gt[omn[1:20], c(12:13,17,18)]
##
## Missense_Mutation Nonsense_Mutation Silent Splice_Site
## TTN 79 8 20 0
## APC 9 65 0 1
## TP53 33 8 1 1
## KRAS 37 1 0 0
## MUC16 31 2 12 0
## SYNE1 21 2 6 0
## DNAH10 19 1 2 0
## DNAH5 18 2 2 0
## LRP1B 19 1 4 0
## ABCA13 17 0 1 0
## NEB 14 3 2 0
## ZFHX4 16 1 5 0
## AHNAK2 15 1 1 0
## FAT4 16 0 5 0
## HMCN1 14 2 1 0
## HYDIN 13 3 5 0
## PCLO 13 3 4 0
## PKHD1 16 0 0 0
## SACS 15 1 4 0
## DNAH11 12 2 4 0
The fact that KRAS and TP53 are in this list gives another crude sanity check.
Multiomics 101: Can we combine the mutation and clinical data?
It isn’t straightforward because sample identifiers are not shared.
clin[1:4,1:3]
## Composite Element REF years_to_birth vital_status
## tcga.af.2687 value 57 0
## tcga.af.2689 value 41 1
## tcga.af.2690 value 76 1
## tcga.af.2691 value 48 0
mut[1:4,c(1,16)]
## Hugo_Symbol Tumor_Sample_Barcode
## 1 OR5B12 TCGA-AG-3586-01A-02W-0831-10
## 2 KRT17 TCGA-AG-3586-01A-02W-0831-10
## 3 SLC10A4 TCGA-AG-3586-01A-02W-0831-10
## 4 C7orf58 TCGA-AG-3586-01A-02W-0831-10
We’ll guess that the following transformation produces the appropriate identifier for the mutation data.
mid = tolower(substr(mut[,16],1,12))
mid = gsub("-", ".", mid)
mean(mid %in% rownames(clin))
## [1] 1
mut$sampid = mid
Let’s simply summarize the total mutation burden per individual.
nmut = sapply(split(mut$sampid, mut$sampid),length)
nmut[1:4]
## tcga.af.2689 tcga.af.2691 tcga.af.2692 tcga.af.3400
## 64 73 45 33
length(nmut)
## [1] 69
dim(clin)
## [1] 171 23
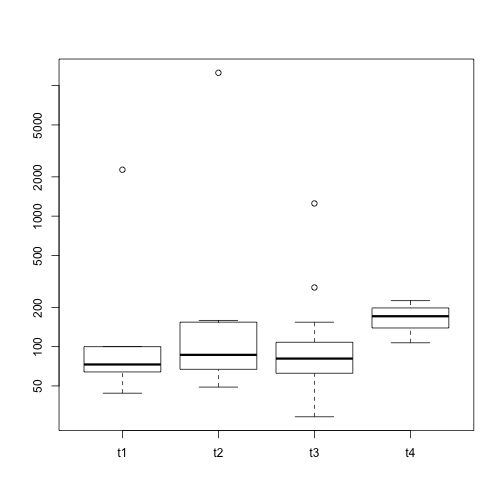
We see that not all individuals with clinical data had a mutation study. We’ll subset the clinical data and visualize the distribution of mutation counts by tumor stage.
clinwmut = clin[names(nmut),]
clinwmut$nmut = nmut
with(clinwmut, boxplot(split(nmut, t_stage), log="y"))
The expression data
There is no experiment-level metadata shipped with the data, but we understand that this is illumina hiseq RNA-sequencing with transcript abundance estimation via RSEM. How the data were transformed to gene level needs to be investigated.
rnaseq = getData(readData, "RNASeq2GeneNorm")
rnaseq[1:4,1:4]
## TCGA-AF-2687-01A-02R-1736-07 TCGA-AF-2689-11A-01R-A32Z-07
## A1BG 20.1873 43.4263
## A1CF 51.0856 313.3531
## A2BP1 0.4257 18.9911
## A2LD1 90.2639 92.2611
## TCGA-AF-2690-01A-02R-1736-07 TCGA-AF-2691-11A-01R-A32Z-07
## A1BG 56.4619 35.9451
## A1CF 24.9913 218.1571
## A2BP1 0.9256 22.6758
## A2LD1 164.3365 113.6528
Again we’ll have to transform the sample identifier strings.
rid = tolower(substr(colnames(rnaseq),1,12))
rid = gsub("-", ".", rid)
mean(rid %in% rownames(clin))
## [1] 1
colnames(rnaseq) = rid
Sadly there is not much overlap between mutation and expression data.
intersect(rid,mid)
## [1] "tcga.af.2689" "tcga.af.2691" "tcga.af.2692" "tcga.af.3400"
## [5] "tcga.ag.3902"
We note some duplicated transformed identifiers; it is not obvious which of the duplicates to keep, so we drop the second.
which(duplicated(colnames(rnaseq)))
## [1] 11 21 23 25 27 35 104
pairs(log2(rnaseq[101:200,c(10:11,20,21,22,23,50,55)]))
rnaseq = rnaseq[,-which(duplicated(colnames(rnaseq)))]
Let’s create an ExpressionSet:
library(Biobase)
readES = ExpressionSet(log2(rnaseq+1))
pData(readES) = clin[sampleNames(readES),]
readES
## ExpressionSet (storageMode: lockedEnvironment)
## assayData: 20501 features, 98 samples
## element names: exprs
## protocolData: none
## phenoData
## sampleNames: tcga.af.2687 tcga.af.2689 ... tcga.g5.6641 (98
## total)
## varLabels: Composite Element REF years_to_birth ... t_stage (23
## total)
## varMetadata: labelDescription
## featureData: none
## experimentData: use 'experimentData(object)'
## Annotation:
There is one individual here with missing tumor stage, and we will simply eliminate that individual.
readES = readES[,-97]
Relating tumor stage to gene expression variation
We’ll use a very crude categorical approach and an alternative will be explored in exercises. We’ll use moderated F tests to test the null hypothesis of common mean expression across tumor stages.
library(limma)
##
## Attaching package: 'limma'
## The following object is masked from 'package:BiocGenerics':
##
## plotMA
mm = model.matrix(~t_stage, data=pData(readES))
f1 = lmFit(readES, mm)
ef1 = eBayes(f1)
## Warning: Zero sample variances detected, have been offset away from zero
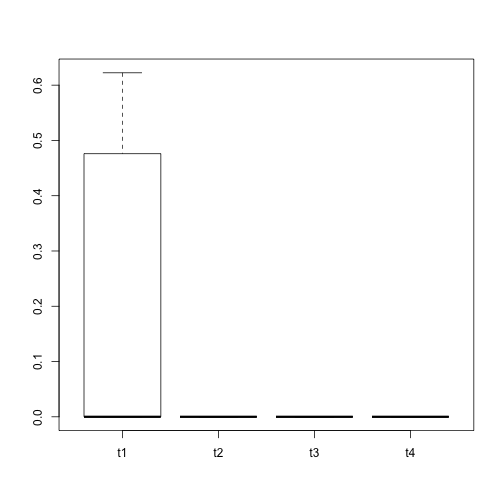
topTable(ef1, 2:4)
## t_staget2 t_staget3 t_staget4 AveExpr F
## LOC100128977 -0.2196981 -0.2196981 -0.2196981 0.01132465 16.060640
## CELA2B -0.4924981 -0.4856924 -0.3966889 0.04003472 14.236630
## GDEP -0.9003575 -0.8376533 -0.8484637 0.09571759 11.852702
## FOLR4 -0.6721456 -0.5992014 -0.5847564 0.09479200 10.805454
## C18orf26 -0.3168032 -0.3042073 -0.3168032 0.02516016 10.417589
## GFRA4 -0.2622521 -0.3289370 -0.3481737 0.04383371 10.269518
## TAS2R41 -0.2117273 -0.2051461 -0.2117273 0.01552745 10.213776
## SEMA5B 1.7503244 1.7161443 2.4269386 4.52996758 9.131087
## CTRB2 -1.5604625 -1.5221324 -1.3959781 0.15557817 8.983471
## TXNDC17 -0.7046034 -0.9283568 -1.4545590 9.81107126 8.216157
## P.Value adj.P.Val
## LOC100128977 1.660698e-08 0.0003404596
## CELA2B 1.010504e-07 0.0010358169
## GDEP 1.187769e-06 0.0081168181
## FOLR4 3.647543e-06 0.0186945693
## C18orf26 5.561914e-06 0.0203603949
## GFRA4 6.539887e-06 0.0203603949
## TAS2R41 6.951991e-06 0.0203603949
## SEMA5B 2.311338e-05 0.0592309205
## CTRB2 2.728557e-05 0.0621535018
## TXNDC17 6.519177e-05 0.1336496376
boxplot(split(exprs(readES)["LOC100128977",], readES$t_stage))
Introducing the 450k methylation data
The 450k data has complicating conditions in common with the expression data – identifiers in need of transformation, duplicates, and only partial match to clinical data.
me450k = getData(readData, "Methylation", 2)
fanno = me450k[,1:3]
me450k = data.matrix(me450k[,-c(1:3)])
med = tolower(substr(colnames(me450k),1,12))
med = gsub("-", ".", med)
mean(med %in% rownames(clin))
## [1] 1
sum(duplicated(med))
## [1] 8
todrop = which(duplicated(med))
me450k = me450k[,-todrop]
med = med[-todrop]
colnames(me450k) = med
ok = intersect(rownames(clin), colnames(me450k))
me450kES = ExpressionSet(me450k[,ok])
pData(me450kES) = clin[ok,]
fData(me450kES) = fanno
me450kES = me450kES[,-which(is.na(me450kES$t_stage))]
Do the 450k measures of DNA methylation associated with gene g correlate with variation of expression of g? We need to synchronize the two datasets.
ok = intersect(sampleNames(me450kES), sampleNames(readES))
meMatch = me450kES[,ok]
esMatch = readES[,ok]
Given these definitions, we can write a helper function
corv = function (sym, mpick = 3)
{
mind = which(fData(meMatch)[, 1] == sym)
if (length(mind) > mpick)
mind = mind[1:mpick]
eind = which(featureNames(esMatch) == sym)
dat = cbind(t(exprs(meMatch)[mind, , drop = FALSE]), t(exprs(esMatch)[eind,
, drop = FALSE]), t_stage = jitter(as.numeric(esMatch$t_stage)))
bad = apply(dat, 2, function(x) all(is.na(x)))
if (any(bad))
dat = dat[, -which(bad)]
pairs(dat)
}
corv("ZNF300") # learned about it from firebrowse.org
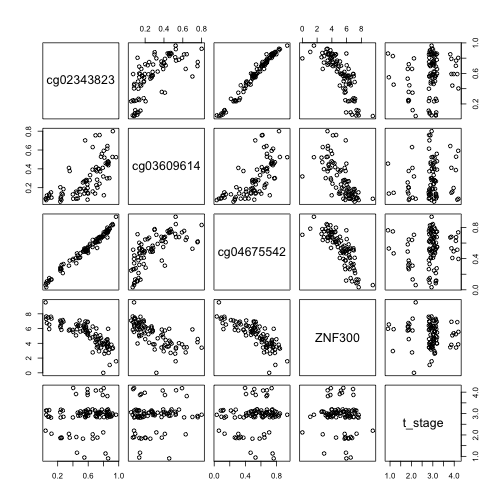
Conclusions
TCGA is an obvious candidate for infrastructure development to support
multiomic analysis.
The MultiAssayExperiment and
curatedTCGAData packages are recent
contributions that address this task.
We have seen some of the challenges that arise
when even a nicely developed tool like RTCGAToolbox is used to acquire the data:
we must be alert to mismatched sample identifier labels, missing data,
inadequate documentation of sample provenance and assay conduct, and so on.
Human effort is invariably required; standards for data quality must go beyond
numerical accuracy and address transparency and usability.
The curatedTCGAData package does employ manually curated
representations, so that the harmonization tasks demonstrated
in this section are not necessary. But in general we need to
know how to harmonize, so these tasks are retained in this course.
By suitably varying code snippets in this document, you can get access to multiomic data of additional modalities (including microRNA, copy number variation, and proteomics). As you discover new approaches to interpreting these measures to create biological insight, please communicate them to the world by adding packages or workflows to Bioconductor.